Clipart tagged: ‘electrolysis’
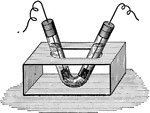
!["If the two wires from a battery be placed in pure water, it will be foud tat practically no current passes...If a small quantity of sulphuric acid is added to the water, the solution is a good conductor. During the passage of current, bubbles form at the ends of the wires: at the positive electrode (anode)...oxygen appear[s]; at the negative electrode (cathode) there is a rapid evolution of hydrogen." -Brownlee 1907](https://etc.usf.edu/clipart/35400/35447/elec_35447_mth.gif)
Electrolysis
"If the two wires from a battery be placed in pure water, it will be foud tat practically no current…
Electrolysis
How electrolysis may occur in a trolley system. TT is the trolley wire, RR is the track. As shown by…
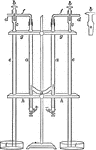
Electrolysis of Hydrochloric Acid
"Hydrochloric acid...is placed in the tubes a. The threeway stopcocks b are turned so that there is…
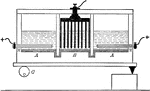
Preparation of Sodium Hydroxide
"The anodes are placed in solutions of salt in compartments AA, and the cathodes in B. A layer of mercury…