Clipart tagged: ‘sulfur trioxide’
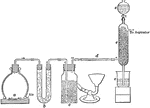
Sulphur Trioxide Preparation
Laboratory preparation of sulphur trioxide, an important step in the process of making sulphuric acid.…

Laboratory preparation of sulphur trioxide, an important step in the process of making sulphuric acid.…