Clipart tagged: ‘sulfuric acid’
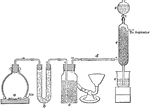
Sulphur Trioxide Preparation
Laboratory preparation of sulphur trioxide, an important step in the process of making sulphuric acid.…

Sulphuric Acid Contract Process
Diagrammatic representation of the sulphuric acid contact process. "Sulphur, or ores containing sulphur,…