Clipart tagged: ‘atoms’
Combinational Volume
"Suppose our volume of hydrogen to unite with the volume of chlorine; if one particle of hydrogen combines…
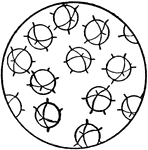
Orientation of Molecules in a Gas or Liquid
This figure shows the orientation of molecules as they would appear in a gas or a liquid. The molecules…
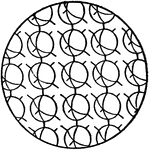
Orientation of Molecules in a Solid
This figure shows the orientation of molecules in a substance in a solid state. The molecules are confined…
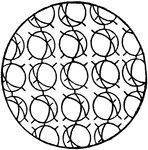
Partial Orientation of Molecules in a Solid
This illustration shows a solid state where the orientation is only partial, as in the case of twin…
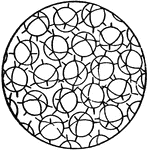
Solid State Showing No Regularity of Molecular Orientation
This figure represents a solid state where there is no regularity of molecular orientation, as in the…