This science ClipArt gallery offers 87 images of lab experiments and discoveries reaffirming principles and concepts in chemistry.
Liquid Agitator
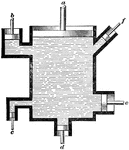
A liquid agitator. Liquid agitators are used with water that has small particles of a substance suspended…
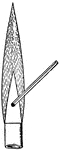
!["Ammonia is a colorless gas with a peculiar odor, lighter than air, and exceedingly soluble in water...The great solubility of this gas is strikingly shown by the 'ammonia fountain.' A flask is filled with dry ammonia, and incerted over water. As soon as the [restraining] clip (not shown in picture) is removed from the ruber tubing, the water rushes in as the gas rapidly dissolves." -Brownlee 1907](https://etc.usf.edu/clipart/35400/35481/ammon_fount_35481_mth.gif)
Ammonia Fountain
"Ammonia is a colorless gas with a peculiar odor, lighter than air, and exceedingly soluble in water...The…
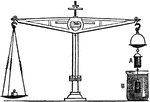
Archimedes Principle
"States that: A body when immersed in a fluid loses exactly as much of its weight as is equal to the…

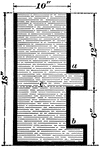
Archimedies Principle
"From one end of a scale-beam, suspend a cylindrical metal bucket, b, with a solid cylinder, a, that…

Archimedies Principle
"It is evident that, when a solid is immersed in a fluid, it will displace exactly its own volume of…
Atmospheric Pressure
"The pressure of the atmosphere may be easily shown by the tube and piston. suppose there is an orifice…
Barometer Measuring Pressure of a Partially Evacuated Vessel
"The degree to which the air has been exhausted from a closed vessel in which there is a partial vacuum…
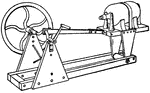
Bucking Board
A bucking board upon which ore is pulverized into manageable pieces with a muller, which is like a hammer.…
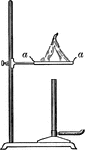
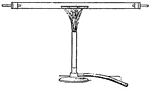
Bunsen Burner
"...gas lighted above an iron gauze (a, Fig. 11) does not catch fire below the gauze because the heat…
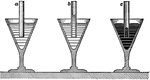
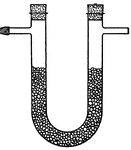
Capillary Attraction
"b is a glass tube in water and c is a glass tube in mercury. The surface of the water in the tube b…
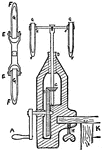
Centrifuge
An older version of a centrifuge. Used to separate dense substances from less dense substances so they…
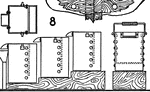
Chlorination Barrel
The chlorination process in gold extraction employs large vats or revolving barrels, the material inside…
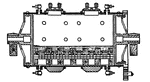
Chlorination Barrel (End Section)
The chlorination process in gold extraction employs large vats or revolving barrels, the material inside…
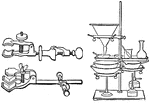
Lab Clamps
The first image is of clamps used in chemistry labs to hold test tubes and other apparatuses. The second…
Combinational Volume
"Suppose our volume of hydrogen to unite with the volume of chlorine; if one particle of hydrogen combines…
Combinational Volume
"When one volume of hydrogen actually unites with one volume of chlorine, two volumes and not one volume…
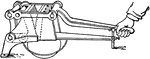
Rock Crusher
A hand-generated rock crusher. Generally used to reduce rock to grainy substance in order to analyze…
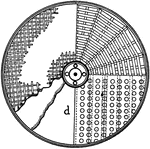
Opposite Currents of Air
"Suppose a b c to represent a portion of the earth's surface a being towards the north pole, c towards…
Cyanide Leaching Tank (Gold Extraction)
In the gold extraction process, finely crushed ores are leached in vats with a very diluted solution…
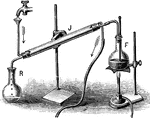
Distilation
"Partly fill with strong brine a Florence glask the cork of which carries a delivery-tube and a thermometer.…
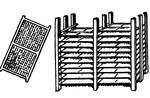
Drying Rack (Glue Manufacturing)
This illustration shows a drying frame and drying rack used in glue manufacturing.
!["If the two wires from a battery be placed in pure water, it will be foud tat practically no current passes...If a small quantity of sulphuric acid is added to the water, the solution is a good conductor. During the passage of current, bubbles form at the ends of the wires: at the positive electrode (anode)...oxygen appear[s]; at the negative electrode (cathode) there is a rapid evolution of hydrogen." -Brownlee 1907](https://etc.usf.edu/clipart/35400/35447/elec_35447_mth.gif)
Electrolysis
"If the two wires from a battery be placed in pure water, it will be foud tat practically no current…
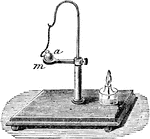
Expansion of Solids
"The expansion of solids by heat is clearly shown by the following experiment: m represents a ring of…

Drop of Milk Showing Fatglobules
An illustration of a drop of milk showing fatglobules, highly magnified.
First Stage in Glue Manufacturing
This illustration shows the first stage in the manufacturing of glue. The material used for making the…
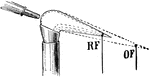
Reducing and Oxidizing Flames
"The reducing flame (as of a blowpipe) is that part of the flame which is deficient in oxygen for combustion…

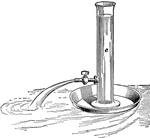
Foutain in Vacuo
"Draw out a piece of glass tubing to a jet, and push it through a perforation in a cork that snugly…
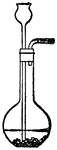
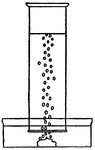
Gas Density
This illustration shows gas with a heavier density then air collecting at the bottom of a beaker.

Mixing Gases in Inverted Containers
"Invert the second vessel over the first, mouth to mouth, so that the contents of the two vessels shall…

Difference of Heat Transference Between Copper and Iron
"Place a bar of iron and a similar one of copper end to end so as to be heated equally by the flame…
Heated Metal
This illustration shows a chemistry experiment where metal is heated to an extreme temperature.
Hydrogen Weight
This illustration shows a test to show the proportions of weight between hydrogen and oxygen.

Hydromechanics
Hydrostatics is a branch of hydrodynamics that deals with the statics of fluids that are usually combined…
Hydromechanics
Water that is placed in an apparatus will have equal pressure if the pistons pushing on it push at the…
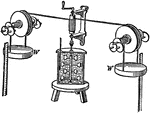
Joule's Experiment
"Joule's experiment on the mechanical equivalent of heat, in which he caused paddlewheels to rotate…
General Law
The general rule of downward pressure is "the pressure upon the bottom of a vessel containing a fluid…
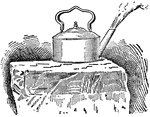
Liquid Air Boiling on a Block of Ice
"Liquid air is air reduced to a liquid form. Liquid air when pure has a bluish tinge, but the liquid…

Liquid in Molds (Glue)
This illustration shows liquid run into molds to cool into jelly in the glue manufacturing process.

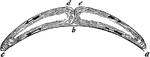
Magdeburgh Hemispheres
"When placed together after the air has been taken out, it will take two very strong men to pull apart."…
Mercury Tube
This illustration shows the proportions of volume in which hydrogen and oxygen combine by introducing…
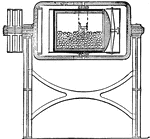
Jar Mill
The interior of a jar mill, which is used to convert hard substances, like the porcelain balls in the…
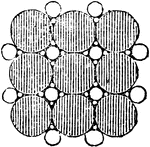
Water with Salt Molecules
"...a certain amount of salt may be successfully dropped into a tumbler brim-full of water without causing…

Old Method of Boiling and Drying (Glue)
This illustration shows the old method of boiling glue in a rope bag, then squeezed dry against a beam.
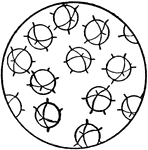
Orientation of Molecules in a Gas or Liquid
This figure shows the orientation of molecules as they would appear in a gas or a liquid. The molecules…
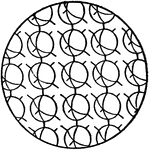
Orientation of Molecules in a Solid
This figure shows the orientation of molecules in a substance in a solid state. The molecules are confined…
Partial Orientation of Molecules in a Solid
This illustration shows a solid state where the orientation is only partial, as in the case of twin…

Pascal's Law
"Pressure exerted anywhere upon a liquid inclosed in a vessel is transmitted undiminished in all direction,…
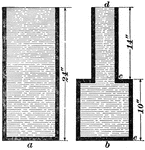
Downward Pressure
In image a, the pressure at the bottom is the same as the pressure at the top of the cylinder. If the…
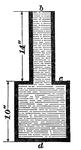
Lateral Pressure
The effects of lateral pressure. The lateral pressures of any two points of the surface of the apparatus…
Upward Pressure
No upward pressure is present on surface c because of because of the weight of the water in part c d.…
!["Get a lamp-chimney, preferably cylindrical. With a diamond or a steel glass-cutter, cut a disk of window glass a little larger than the cross-section of the lamp-chimney. Pour some fine emery powder on the disk, and rub one end of the chimney upon it, thus grinding them until they fit accurately...place [the chimney] under the water as shown. the upward pressure of the water will hold the disk in place. Pour water carefully into the tube; the disk will fall as soon as the weight of the water in the chimney plus the weight of th disk, exceeds the upward pressure of the water." -Avery 1895](https://etc.usf.edu/clipart/35900/35965/water_press_35965_mth.gif)
Water Pressure Experiment
"Get a lamp-chimney, preferably cylindrical. With a diamond or a steel glass-cutter, cut a disk of window…